|
A few of the more beneficial fungi in soil form a symbiotic relationship with roots as mycorrhiza. Ectomycorrhizal fungi tend to grow on the surface of the root tissue and between cells inside the root. These fungi are most common among tree species and often revealed when they form mushrooms around near trees.
Corn and other annual crops often have symbiosis with endomycorrhizal fungi that penetrate the root tissue, initially growing between cells as filaments (hyphae) and then forming special membrane-enclosed structures called haustoria that push against the cellular membranes. This allows efficient movement of minerals such as phosphorus, copper, and zinc from the fungus into the corn root cell and sugar molecules into the fungus. Increased corn root water uptake also is associated with this symbiosis. Many endomycorrhizal fungi produce spores that exist in soil until stimulated to germinate and grow towards roots. They cannot live as saprophytes and are dependent upon on living root tissue. This has made studying these fungi difficult because independent artificial culture is not easy. Most of these fungi are classified as a member of the Glomus genus and several species may be in the same field. There are indications that the same species can infect corn and soybean, suggesting no reductions with crop rotation between these two crops. Apparently, these fungi are more available to early corn infection in no-till than in deep tillage fields. Mycorrhizal fungi are among the unseen environmental factors that contribute to the small variations that occur within the corn field. To state that the environment of the corn seed when planted in spring is complex is an understatement. Physical factors like temperature and water would seem obvious but the interactions with the germinating corn seed and surrounding microbes is not always clear. Some have the capacity to destroy the root tissue and are thwarted by resistance factors in the corn root tissues. A few microbes appear to cause a reaction by the plant that not only results in less root damage from pathogens but cause the root to grow faster.
A strain of the fungal species Trichoderma harzianum designated as T22 has been shown to increase root growth in young plants of several species including corn. Use of this fungus as an inoculant has been shown to be helpful, at least in certain stress field environments. This especially has been true with shrunken2 sweet corn (supersweet), varieties that are known to have considerable less germination percentage than most commercial field corn. It is as if the weakened cells are stimulated to grow, perhaps repairing cell membranous structures, as well as warding off potential pathogens. Use of biologicals such as strains of Trichoderma have been shown to result in larger roots in some early season environments and consequently better uptake of minerals and water. There remains a lot to learn about the interactions between the plant tissue, fungus and environments. I am always impressed by what we know about corn plants and what we don’t know, especially about interactions of corn in its environment. Biology of the corn plant is complex, with genes turned on to produce complex products in timely fashion to result in grain production. Even more complex are the interactions among the microbes surrounding the germinating corn seed in the field. These organisms compete with each other for nutrition from the dead organic matter as well as the live new root tissue. The multiple bacteria species oppose each other chemically and fungi produce antibiotics to gain advantage over bacterial and other fungal species.
Corn plants not only produce defensive chemicals to fight off potential pathogens, but also encourage favorable microbes. One example was described in a recent publication:(PloS ONE Feb. 20,2017) in which the DIMBOA exuded from corn roots not only inhibits soil insects but also favors a beneficial bacterium species, Psuedomonas putida, allowing it to outcompete with potential corn pathogens surrounding the root tissue. DIMBOA is a common biocide produced in corn that not only inhibits insects like corn borers in young stalks it also affects micro-organisms. Some of the bacterial population can fix nitrogen, making nitrogen available to plants but also to other microbes for their metabolism. And there is competition for that as well. One of the advantages of the corn plant comes from the roots quickly going deeper than the upper soil surface where the organic matter and microbes are most prevalent. This not only favors high quality seed but also corn genotypes that have fast early growth. That corn field that looks like only a lot of dirt immediately after planting has a lot of unseen activity. 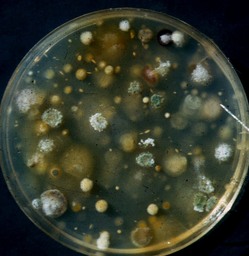 Seeds are surrounded by many microorganisms mostly feeding on organic matter that is mostly dead. The photo, copied from our publication of Stalk rot of Corn power point, shows the multiple colonies grown from a particle of soil in the spring of a corn field. These organisms are competing for the carbs locked up in the dead debris in the soil. Fusarium species are among these that can also penetrate some living corn roots. Fusarium is a fungus genus composed of many species. This group of fungi asexually produce spores called conidia that move in the wind. Most of these species also have a sexual stage in which the haploid hyphae fuse to form a diploid structure (ascus) in which the nuclei undergo meiosis, producing haploid ascospores, to produce more hyphae with new genetic combinations. The fact that the asexual and sexual stages are separate in time and, sometimes, location adds to the difficulty for specialists to associate the two. The ‘rules’ of naming these fungi declares that the sexual stage is primary. Consequently, on fungus commonly infecting corn seedlings, stalks and ears is known as Fusarium graminearum but also by its sexual stage name of Gibberella zeae, Another common Fusarium infecting seedlings, stalks and ears is Fusarium verticillioides also known for the sexual stage name of Gibberella fujikuroi. Adding to the naming confusion, the previous name for F. verticillioides was Fusarium moniliforme. The names are confusing but biology of these fungi is not much better. They tend to mostly be weak pathogens. They can enter weakened root and mesocotyl tissue of the germinating seed. Host resistance seems to limit the fungus ability to destroy much tissue but it appears to simply live in the corn tissue without causing visible damage. It is not unusual to find at least a few seed germinating on a paper towel that look normal but have some hyphae of Fusarium verticillioides growth. This species frequently can be isolated from corn leaf tissue with no apparent damage. Likewise, Fusarium species are nearly always isolated from stalks of corn plants. Often, if no symptom associated with another pathogen, such as the black streaks in outer rind caused by the anthracnose fungus (Colletotrichum graminicola), we tend to call it Fusarium stalk rot. The death of the stalk mostly was a biological problem of the corn plant and fungi like Fusarium was there, among others, to assist in the digestion of dead stalk tissue. The field environment of corn germination includes many organisms. One group active in early spring are the Oomycetes. These organisms were once classified as fungi but now their distinctiveness has most specialists agreeing that they are more closely related to brown algae. Fungi have chitin cell walls whereas Oomycetes have cellulose walls. Oomycetes have swimming spores, zoospores, whereas this is not a feature of most true fungi. This is the feature that makes Oomycetes genera such as Pythium so significant to corn seedling survival.
Pythium species reproduce with swimming sperm cells fertilizing egg cells, while in infected live or dead plant tissue. These then form a thick-walled oogonium that persists during stress, including winter temperatures. When in water, and spring temperatures in the 50’s, sporangia growing from the oogonia release the swimming zoospores. Attracted to sugars released by primary roots and the mesocotyl of corn seedlings. In some cases, the oogonia produce filaments (hyphae) that infect the roots also. Infection of these tissues can cause the seedlings to die, cutting off water to the emerging leaves. If the seedlings survive this early infection of the primary root and mesocotyl, secondary roots emerging from the crown area bypass the infection and outgrow the damage. Low temperature and oxygen deficiency because of water-soaked heavy soil contribute to the seedling vulnerability to damage. Seeds with previous membrane damage resulting in slow early seedling growth are often the most vulnerable, perhaps because they are slower to produce the more resistant secondary roots. There is evidence that the same Pythium species infecting corn also infect soybeans and several grasses as well. Pythium species do exist in a competitive environment with other microorganisms capable of inhibiting Pythium success. Apparently low oxygen, cool environment of water-soaked heavy soils favor the Pythium species. Seed treatments on corn (and soybeans) are often aimed at not allowing the seedling infection. Races of Pythium are known to overcome some of the treatments. It is unfortunate that genetic variability works for all organisms! When varieties, not hybrids, were common on the USA corn belt, average yields were frequently less than 50 bushel/acre. Part of the reason for the low yields was the lack of uniformity of pollination timing and plant height within the fields, mostly due to genetic variability among plants within the variety. Significance of obtaining genetic uniformity within a field became apparent in the early 1900’s as inbreds were combined to make specific hybrids, although the seed production quantity on the inbreds forced the use of double crosses. As inbred seed yields became more commercially acceptable in the 1960’s, the advantage of single crosses became obvious at least partly because of genetic uniformity among plants in the field.
Variability in plant height, especially when a shorter plant is adjacent to a taller one, regardless if it is due to genetic variability, or uneven emergence, penalizes the performance of the smaller plant. If a corn plant is slower to push out silks than surrounding plants, it will not match the yield of the majority. Producing genetically pure single crosses has several challenges for seed companies. Haploid-di-haploid breeding schemes produce genetically pure, homozygous individual plants. However, the small quantity of seed needs to be increased, initially to make test hybrids and later, if successful to produce commercial hybrids. Each increase carries some risk of contamination that can be mostly controlled by techniques but even with a low mutation rate the eventual inbred line will have the essence of the parent seed but also include some plants slightly different from the original. Seed producers make considerable effort to maintain genetic uniformity in parents and single cross hybrids but evaluation is difficult. DNA analysis methods may identify presence of DNA differences but may have difficulty in realizing the significance. With 32000 genes in corn, the few changes may not matter in important aspects of performance. If a seed production field is contaminated with hybrid, resulting ‘outcrosses’ are distinct from the correct hybrid plants for many phenotypic characters expressed as seedlings as well as mature plants. Pollen from hybrid plants have more than 1024 different genetics from the two hybrid parents. Consequently, outcross plants within a single cross seed production will differ from each other and from the intended single cross for many characters. Several years ago, we intentionally made outcrosses by crossing hybrid pollen with two popular female corn parents. We planted the resulting seed for our standard seedling growout test and later transplanted to grow the plants to maturity. Seedlings expressed the differences as predicted. Mature outcross plant showed considerable variation in plant height, flowering time, silk color and tassel shape as predicted. If the outside pollen was placed on a female in which the normal male was short, more of the outcrosses were taller than the expected hybrid. If placed on a female in which the correct male was tall, very few of the outcrosses were taller than the correct hybrid. In other words, a higher percentage of outcrosses were taller than the canopy if the correct male was short. In both cases, a few plants were very short and inferior. Ultimate field performance of a hybrid is affected by any factors affecting uniformity. Genetics is one, but germination quality uniformity and multiple micro-environmental factors also are large. Seed producers attempt to limit negative effects of the first two. Among the challenges facing seed producers and their customers is predicting the effective germination of the seed in the field. Dormant seed remains alive, maintaining the membranes of the many cell organelles at a low rate. Membrane integrity is essential to the increased metabolism needed for germination. Physical and time factors in the journey from when the corn seed is forming in the field, through storage at the grower’s farm all can have some effect on the eventual uniform emergence in the field the following spring. Predicting the final germination, the slope of aging in the seed after harvest is the effort of seed testing.
Standard warm tests are done at 70°F in a uniformly moist substrate. Cold tests are usually performed with a week at 50° and then 4-7 days at 70°. The warm test basically identifies the individual seed that have deteriorated beyond repair even at a temperature that would allow normal recovery of damage whereas the low temperature of the cold test inhibits the metabolism needed to repair the membranes as they swell from water imbibition. Often seed producers test seed immediately after drying and shelling and then later after adding treatment and bagging. Tests done at these two times can allow an estimation slope in which the seed is aging or deteriorating (Seed Testing info). A few factors become critical in correctly predicting the germination percentage at the time of planting the next spring. Sampling to represent the units of a lot that are sold is a potential problem that is usually met automatically by seed producers but still requires care. A more difficult problem comes when an environmental interaction in the production field causes cellular stress that don’t show until a few months before the seed is planted the following spring. Seed producers are faced with the difficult problem of timely distribution of the seed in a relatively short time and yet potentially testing the seed a few months before it is planted in the field. Genetics also seem to add to the problem as some female parent genetics tend to deteriorate quicker than others. Adding to the confusion, each germination lab has slight differences in results, even if all attempt to follow nearly the same methodology. Slight differences in defining the slower germinating seed contributes to part of the problem but also even attempts to have identical methods seem to be difficult. Ideally a lab attempts to be consistent and a seed producer attempts to adapt their in-house decisions on marketing seed lots with some field data comparisons with germinations. Even then, the field studies need multiple reps because of usual field soil variabilities. Ever notice that despite our desire for things to be simple, it rarely happens that way? We want to think of all single cross seed in a bag are the same, but they are not identical genetically or in germination quality. Even with multiple generations of selfing in development of the parent seed, some mutations occur with each generation of seed increase prior to planting in the hybrid seed field. Most often these mutations are non-consequential to hybrid performance and especially not visible in the field where many small environmental effects are affecting appearance of the plants. The closer we get to discerning DNA differences the more difficult it becomes to distinguish inconsequential differences from the drastic ones.
Seed quality differences among the seed in that one bag of hybrid seed also shows differences. A warm test may show 95% germination but even beyond the non-germinating ones, there will be some that are slower to germinate than others even when all environment is uniform. As the percent germinated gets lower, more late ones become evident. A cold test, especially like ours at Professional Seed Research, Inc. in which we cover the seed with 3/4 inch of artificial soil, nearly always show lower percent germination and more late emerging plants than the warm test. Why are all the seed not with the same quality when produced in same field? Unfortunately, not all the seed on a single seed field ear have the same environment. First silk, coming from the ovules near the base of the ear, emerge 3-6 days before the final silk. Successful pollination by the correct male parent is dependent of many variables, including factors associated with maturity for the male and female in the seed field. In general, pollen timing is affected more by accumulation of heat units whereas silking is favored by water. Cool wet pre-flowering weather can lead to silks being exposed before pollen. This not only makes the seed more vulnerable to contamination by outside pollen from field corn, resulting in outcrosses, but also to infection from fungi such as Fusarium or Diplodia species traveling down the silk channel before it closes after pollination. The opposite can happen with hot dry weather, in which the silk emergence is delayed, causing the pollen to be spent before all the silk emerges. Consequently, there often are differences in germination (and outcrosses) among seed positions on the ear. We want it to be simple but that rarely occurs in biology of seed- and that makes it interesting for some of us! |
About Corn JournalThe purpose of this blog is to share perspectives of the biology of corn, its seed and diseases in a mix of technical and not so technical terms with all who are interested in this major crop. With more technical references to any of the topics easily available on the web with a search of key words, the blog will rarely cite references but will attempt to be accurate. Comments are welcome but will be screened before publishing. Comments and questions directed to the author by emails are encouraged.
Archives
December 2021
Categories
|
 RSS Feed
RSS Feed